Trusted company that deals in quality-made products


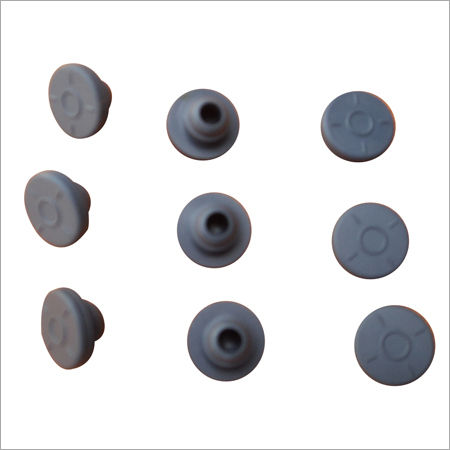
Sterilized Rubber Closures
1.00 - 10.00 INR
Product Details:
- Size Available in multiple sizes to fit various container neck diameters, e.g., 13 mm, 20 mm, 32 mm.
- Rubber Type Bromobutyl rubber, Chlorobutyl rubber.
- Surface Finish Smooth, matt finish to ensure proper sealing and sterility.
- Thermal Conductivity Low (approx. 0.13 W/mK).
- Product Type Sterilized Rubber Closure
- Tolerance 0.2 mm
- Material Pharmaceutical grade bromobutyl or chlorobutyl synthetic rubber.
- Click to View more
X
Sterilized Rubber Closures Price And Quantity
- 100000 Piece
- 1.00 - 10.00 INR
- Meets USP Class VI requirements for pharmaceutical rubber.
- 10 (as validated by manufacturer).
- 5 years when stored as per recommended conditions.
- Tested and certified for low endotoxin levels.
- Compatible with glass and plastic injection vials.
- Gamma irradiation or autoclaving as per pharma standards.
- Passed compliance with global pharmacopeia extraction standards.
Sterilized Rubber Closures Product Specifications
- Sterilized Rubber Closure
- Smooth, matt finish to ensure proper sealing and sterility.
- Low (approx. 0.13 W/mK).
- High resistance to chemical, microbial, and environmental factors.
- 13 mm, 20 mm, 32 mm or as specified.
- Not greater than 1.5% (typical specification for pharmaceutical rubber closures).
- 0.2 mm
- Single molded piece; may include central puncturable region.
- Pharmaceutical grade bromobutyl or chlorobutyl synthetic rubber.
- No; made from pharmaceutical grade synthetic rubber.
- Below 1% (low water absorption).
- Closure / Sealing Component
- Grey
- 20% (typical for rubber closures).
- Less than 1%.
- Used for sealing vials, bottles, and containers in pharmaceutical and laboratory settings to maintain sterility.
- Varies according to size specification; generally ranges from 20 mm to 32 mm.
- Bromobutyl rubber, Chlorobutyl rubber.
- Available in multiple sizes to fit various container neck diameters, e.g., 13 mm, 20 mm, 32 mm.
- Negligible (rubber formulations are primarily hydrocarbon polymers).
- Flat or slotted top, with or without central hole depending on application.
- Meets USP Class VI requirements for pharmaceutical rubber.
- 10 (as validated by manufacturer).
- 5 years when stored as per recommended conditions.
- Tested and certified for low endotoxin levels.
- Compatible with glass and plastic injection vials.
- Gamma irradiation or autoclaving as per pharma standards.
- Passed compliance with global pharmacopeia extraction standards.
Sterilized Rubber Closures Trade Information
- 100000 Piece Per Week
- 15 Days
- All India
Product Description
We are counted among the most distinguished organizations that are engaged in manufacturing, supplying and trading high quality Sterilized Rubber Closures. These products are manufactured using the best quality rubber available in the market. Our products are mostly demanded by hospitals, laboratories and many other places as these products provides perfect seal and is easy to use. We are able to offer these Sterilized Rubber Closures in various sizes and colors to meet our clients' requirements.
Product Specification
Material | rubber |
Type | Rubber Stopper |
Diameter | 13 to 33mm |
Color | Grey, Red |
Brand | Shyamwell Pack |
Shape | Round |
Premium Pharmaceutical Rubber Closures
Designed for reliability in critical settings, these sterilized rubber closures offer seamless compatibility with various glass and plastic injection vials. Engineered for ease of puncture and secure resealing, each unit meets stringent international pharmacopeia and USP Class VI guidelines. Their low ash, nitrogen, and volatile content underscores their exceptional purity, while durable synthetic rubber ensures consistent performance over extended shelf life.
Strict Sterilization and Compliance
Closures are sterilized using validated autoclaving or gamma irradiation processes to achieve a sterility assurance level of 10. Rigorous testing guarantees low endotoxin levels, minimal extractables, and compliance with global extraction standards. Customers can rely on trouble-free integration into both pharmaceutical and laboratory packaging workflows.
FAQs of Sterilized Rubber Closures:
Q: How are these rubber closures sterilized to meet pharmaceutical standards?
A: Sterilized Rubber Closures undergo either gamma irradiation or autoclaving, both of which are internationally accepted methods for achieving sterility in pharmaceutical products. Each batch is validated to a sterility assurance level of 10, ensuring the closures are safe for critical medical and laboratory use.Q: What types of vials or containers are these closures compatible with?
A: These closures are specifically designed for compatibility with glass and plastic injection vials in a range of diameters (13 mm, 20 mm, 32 mm), making them versatile for various pharmaceutical and laboratory container types. Their precise design ensures a secure seal and optimized performance.Q: When should I use sterilized rubber closures instead of non-sterilized options?
A: Sterilized rubber closures are essential whenever maintaining sterility of the container content is critical, especially in pharmaceutical filling and laboratory testing. Use them for injectable medications, sensitive reagents, and any applications requiring strict aseptic conditions.Q: Where are these closures commonly found in use?
A: These closures are widely used by pharmaceutical manufacturers, research laboratories, and healthcare institutions across India and globally. Their high compliance and reliability have made them the preferred sealing choice for injectable vials and other sterile containers.Q: What benefits do pharmaceutical synthetic rubber closures provide compared to natural rubber?
A: Pharmaceutical-grade synthetic rubber, such as bromobutyl or chlorobutyl, offers higher chemical resistance, reduced allergenicity, lower water absorption, and greater consistency than natural rubber. This makes them safer and more durable for pharmaceutical sealing applications.Q: How do I ensure the proper fit and function of these closures for my application?
A: Select the closure size that matches your container neck diameter (e.g., 13 mm, 20 mm, 32 mm). The closures are available with flat or slotted tops, and optional central holes depending on application needs. Tolerance is maintained at 0.2 mm for a secure, leak-proof seal.Q: What makes these closures compliant with global pharmacopeia standards?
A: These closures meet USP Class VI requirements, pass global extraction and endotoxin tests, and maintain strict limits on ash, volatile content, and other impurities. Each is manufactured and validated according to international pharmacopeia standards, ensuring safety and reliability for critical uses.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email